Complete and Balance the Following Half-reaction in Acidic Solution Mno4-
1 Calculate the pH of a solution prepared by dissolving 145 g of sodium acetate CH3COONa in 675 mL of 020 Macetic acid CHCOOHaq. Find step-by-step Chemistry solutions and your answer to the following textbook question.

Solved Complete And Balance The Following Redox Reaction In Acidic Solution Reo4 Aq Mnoz S Re S Mno4 Aq Aq Hio Re H Mn Oh Hzo H Reset X Hzo Delete
MnO 4 --- Mn 2 It is to be balanced in acidic solution.

. Here is a second half-reaction. Complete and balance the following half-reaction in acidic solution MnO4 aq MnOz s 0 of 1 point earned 3 attempts remaining Complete and balance the following half-reaction in basic solution S2 aq SO2 g O of 1 point earned 3 attempts remaining Complete and balance the following redox reaction in acidic solution 103-. We review their content and use your feedback to keep the quality high.
BALANCE THE REDOX REACTION USING HALF CELL METHOD IN AN ACIDIC MEDIUM CHCl3 MnO4- Cl2 CO2 Mn2. Complete and balance the following redox reaction in acidic solution H2O2aq Cr2O2-aq O2g A. Redox reactions are those in which one of the molecules is.
Balancing a redox reaction in basic aqueous solution in ten easy steps. 4MnO4- aq 5CH3OH aq 11H2O l 12H aq 4Mn2 aq 5HCO2H aq can someone please help. We review their content and use your feedback to keep the quality high.
SO3 2- H2O _ SO4 2- _. First Write the Given Redox Reaction. MnO4 H2S Mn2 S acidic medium.
ChemistryQA LibraryComplete and balance the following half-reaction in acidic solution MnO4-aq. Complete and balance the following equation. Question 3 of 16 Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s Previous question Next question.
Complete and balance the following half-reaction in acidic solution Ass H2ASO4 aq 04- O3- n2- 02 3 D4 3 5 7 8 O2 D4 Do 8 s 1 g aq OH As H. Use e as the symbol for an electron. MnO₄ I ----- MnO₂ I₂.
Use H to balance H. Volume change upon dissolving the sodium acetate is negligible. Chemistry questions and answers.
Complete and balance the following half-reactions. Identify Oxidation and Reduction half Reaction. Click hereto get an answer to your question UN Complete and balance the following equation.
Experts are tested by Chegg as specialists in their subject area. Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s Complete and balance the following half-reaction in basic solution 103- aq 10- aq. MnO4 - _ MnO2 2 H2O _.
Balance the following half reaction in acid solution. MnO4 aq CH3OH aq Mn2 aq HCO2H aq acidic solution I KNOW ITS a redox so i followed the steps and got. MnO4 - _ MnO2 _.
The balanced equation for the reaction in acidic solution is 14 Haq 2 Mn2aq 5 NaBiO3s 2 MnO4aq 5 Bi3aq 5 Naaq 7 H2Ol There. U4 -- UO2 There is a hint. Click hereto get an answer to your question For the redox reaction MnO4- C2O42 - H Mn2 CO2 H2O the correct coefficients of the reactants for the balanced equation are.
Complete and balance the following half-reaction in basic solution. SO3 2- _ SO4 2- _. Experts are tested by Chegg as specialists in their subject area.
Complete and balance each of the following half-reactions steps 25 in half-reaction methodMnO4aq Mn2aq in acidic solutionOpenStax is a registe. Sign In to Writing Essays Science. Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s 3 04- 2- D- 2 31 4 1 2 3 4 4 5 6 7 8 8.
In acidic solution you may add H2O and H as needed to balance oxygen and hydrogen. Who are the experts. Here is the half-reaction to be considered.
MnO₄ ----- MnO₂ Reduction I -----I₂ Oxidation Step3. MnO4- aq C2O2-2 aq - MnO2 s CO2 g Expert Answer. Balance the atoms undergoing change in the Oxidation number.
Operatorname Sn 2 a q longrightarrow operatorname Sn 4 a q acidic solution. TO produce a balanced equation we adds i and ii in such a way as to remove the. Ask an expert Ask an expert done loading.
Who are the experts. In each case indicate whether the half-reaction is an oxidation or a reduction. MnO4Mn2aq MnO4Mn23e MnO48H5eMn24H2O MnO48HMn24H2O MnO48HMn24H2O5e none of the above.
Sign In to Tutor. Separate into Half Equations. Cr 2 O 7 2 --- Cr 3 acidic soln As I go through the steps below using the first half-reaction try and balance the second half-reaction as you go from step to step.
M nO 4 8H 5e M n2 4H 2Ol ii For each half-equation charge and mass are balanced ABSOLUTELY and thus it reflects stoichiometry. Sign In to Solutions. MnO4 - 4 H _ MnO2 2 H2O _.
Use water to balance O. 9 0 Oz 03. H H2O e H30 Reset x H2O Delete 4- 2.

Solved Complete And Balance The Following Redox Reaction In Chegg Com
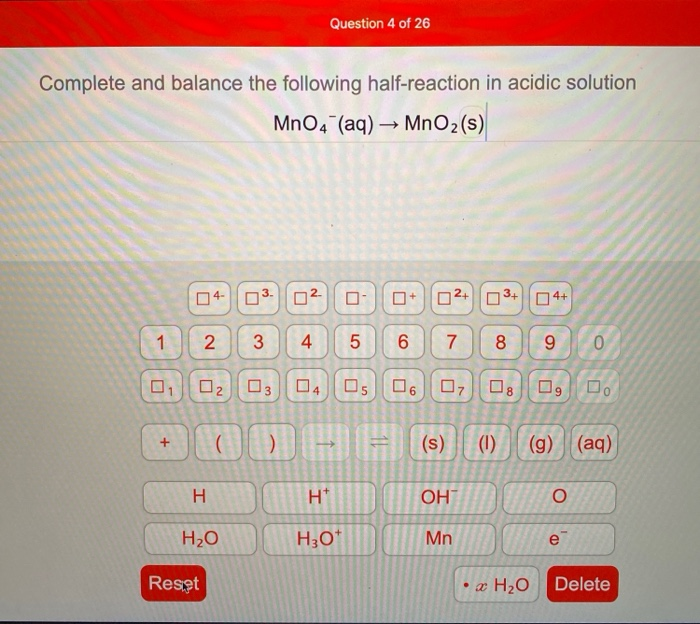
Solved Question 4 Of 26 Complete And Balance The Following Chegg Com
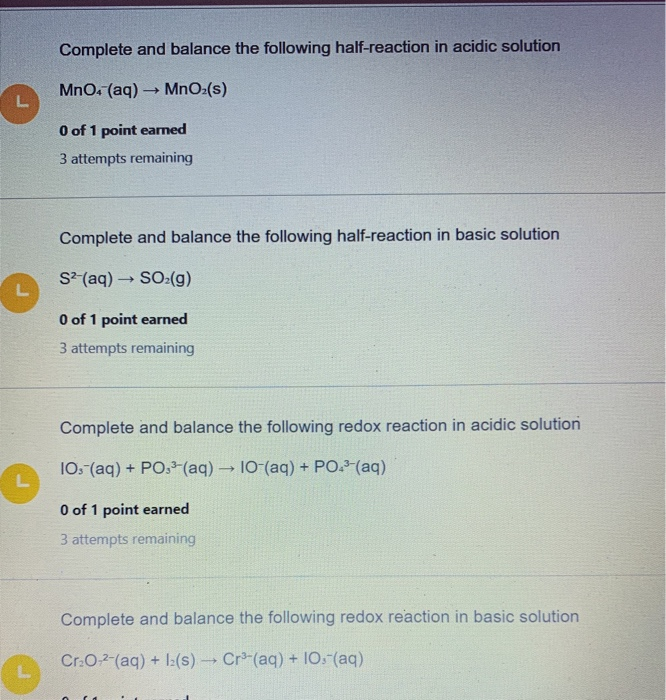
Solved Complete And Balance The Following Half Reaction In Chegg Com
Comments
Post a Comment